de novo biosynthesis of steviolglycosides sweetener in Escherichia coli
Plants derived isoprenoids are an important source of natural pharmaceuticals, fragrances and food additives. Since the yield of the rare isoprenoids in native plants is extremely low and semi-synthesis is inefficient, development of microbial synthetic biology has been revolutionizing the industrial production of important plant terpenoids by reconstructing the downstream biosynthetic pathways and systematically engineering the supply of common terpenoid precursors.
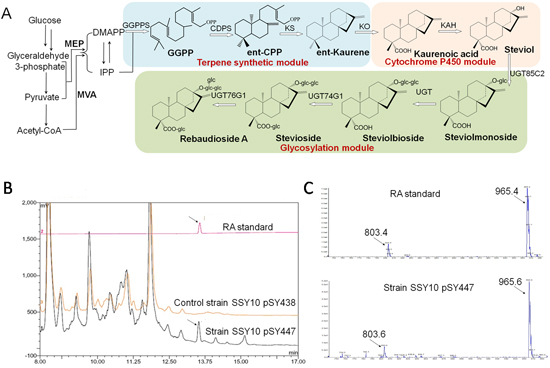
The diterpenoid steviolglycosides (SGs) from Stevia rebaudiana are intense natural sweeteners with various healthy benefits. The efforts in constructing chassis cells for de novo biosynthesis of SGs have been suffocated by lack of certain enzymes with proper activities in bacterial hosts. Dr. Yong Wang and his colleagues identified a novel kaurenoic acid 13α-hydroxylase (KAH) designated as KAHn2 based on mining of the RNA-seq data and characterized a UDP-glucose transferase (UGT) designated UGT91D2 with the activity of steviol-13-monoglucoside-1,2-glucosylase in the E. coli. Based on this, the intermediate metabolites for SGs biosynthesis were assembled and tested step wisely. Through improving the precursors supply, the yield of kaurene and kaurenoic acid reached 194.12 mg l-1 and 100 mg l-1, respectively. However, only 0.605 mg l-1 of steviol was produced and the membrane-associated cytochrome P450 enzyme KAH was identified as the rate-limiting step. To relieve this bottleneck, an engineered CYP714A2 from Arabidopsis thaliana replacing KAHn2 significantly improved the yield of steviol to 15.47 mg l-1. Furthermore, by subsequently incorporating the UGT module, 10.03 mg l-1 of rebaudioside A, the major component of native stevia extracts, was successfully produced in shake flask among various kinds of SGs. This de novo SGs biosynthetic pathway constructed in E. coli chassis employing novel enzymes identified, characterized or modified in this study is another successful case of cell factory for nature product production, which forms a base for massive production of various SGs components via synthetic biology manufacturing.
The study entitled “Pathway mining-based integration of critical enzyme parts for de novo biosynthesis of steviolglycosides sweetener in Escherichia coli” has been published online in Cell Research on Sep 11, 2015.
The research was supported by Ministry of Science and Technology, NSFC and Shanghai Science and Technology Committee.
CONTACT:
Yong Wang, PhD
Key Laboratory of Synthetic Biology
Institute of Plant Physiology and Ecology
Shanghai Institutes for Biological Sciences
Chinese Academy of Sciences
Shanghai 200032, China
Tel/Fax: 54924295
E-mail: yongwang@sibs.ac.cn